What Is The Difference Between Electron Affinity And Ionization Enthalpy?
The Periodic Table: Atomic Radius, Ionization Energy, And Electronegativity
Keywords searched by users: What is the difference between electron affinity and ionization enthalpy difference between ionization energy and electron affinity with example, difference between ionization energy and electron affinity and electronegativity, difference between ionization energy and electronegativity, relation between ionization energy and electron affinity and electronegativity, difference between electron affinity and electronegativity, electron affinity trend, ionization energy and electron affinity equation, similarities between ionization energy and electron affinity
What Is The Difference Between Affinity And Enthalpy?
Certainly! Here’s a revised paragraph that provides a clearer explanation of the difference between affinity and enthalpy related to electron behavior:
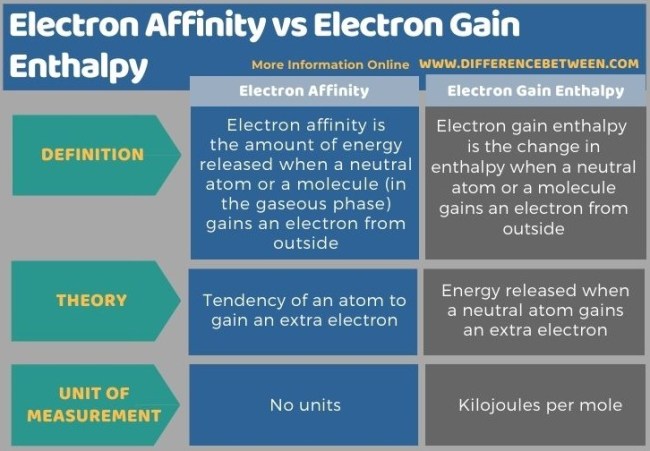
In the realm of electron behavior, it’s essential to distinguish between two closely related concepts: electron gain enthalpy and electron affinity. These terms pertain to the interactions of isolated gaseous atoms with electrons. Electron gain enthalpy refers to the amount of energy released when an isolated gaseous atom accepts an additional electron into its electron cloud. This process represents the energy change associated with the electron’s acquisition. On the other hand, electron affinity is a broader concept that describes the inherent tendency of an isolated gaseous atom to attract or accept an electron. It encompasses both the energetic aspect (enthalpy) and the atom’s natural inclination to acquire an electron. In summary, while electron gain enthalpy quantifies the energy change when an atom gains an electron, electron affinity encapsulates this energy change and the atom’s propensity to accept electrons in general. So, electron affinity encompasses both the energetic and natural inclination aspects of electron acceptance by isolated gaseous atoms.
What Is The Relationship Between Electron Affinity And Ionization Energy?
The relationship between electron affinity and ionization energy is closely tied to the periodic table’s organization. Both of these atomic properties exhibit consistent trends across the table. As you move along a period from left to right, both ionization energy and electron affinity generally increase. This means that it becomes more difficult to remove an electron from an atom, and atoms have a greater tendency to accept additional electrons. Conversely, as you move down a group or column, both ionization energy and electron affinity typically decrease. This trend can be attributed to the shielding effect, which reduces the attraction between the outermost electrons and the nucleus as you move further away from the nucleus. Therefore, on January 29, 2023, it is essential to understand that ionization energy and electron affinity exhibit parallel trends on the periodic table, with both increasing along periods and decreasing down groups due to the shielding effect.
Share 18 What is the difference between electron affinity and ionization enthalpy






Categories: Top 13 What Is The Difference Between Electron Affinity And Ionization Enthalpy
See more here: b1.brokengroundgame.com
Ionization energy (IE) is the energy required to remove an electron away from an atom and electron affinity is the energy released when an electron is added to a valence shell of the atom.Ans: The main difference between electron gain enthalpy and electron affinity is that electron gain enthalpy is the amount of energy released when an isolated gaseous atom accepts an electron whereas electron affinity is the tendency for the isolated gaseous atom to accept the electron.Both ionization energy and electron affinity have similar trend in the periodic table. For example, just as ionization energy increases along the periods, electron affinity also increases. Likewise, electron affinity decreases from top to bottom due to the same factor, i.e., shielding effect.
Learn more about the topic What is the difference between electron affinity and ionization enthalpy.
- Ionisation energy and electron affinity are defined … – Vedantu
- Electron Gain Enthalpy: Types, Electron Affinity & Exceptions
- Ionization Energy – Chemistry LibreTexts
- EC Test Review Flashcards – Quizlet
- Is the magnitude of ionisation enthalpy and ionisation energy is same?
- Difference Between Electronegativity and Electron Affinity – Vedantu
See more: blog https://b1.brokengroundgame.com/media

